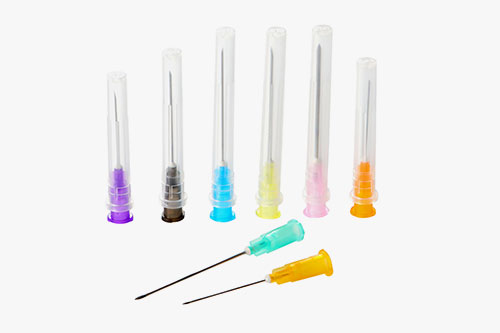
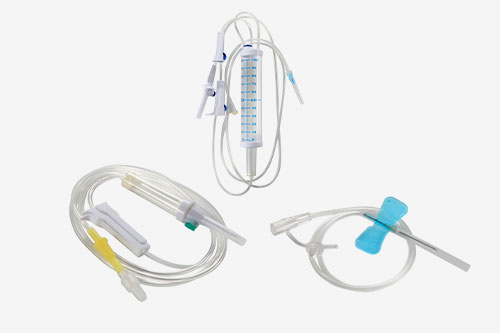
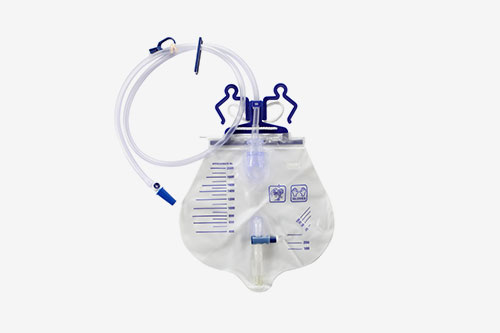
About Us
Our founder Mr Zou Wenqing,started the disposable plastic syringe production,it's the earlist plastic syringe production line in China,but the workshop is not 100000 class medical grade.Before 1985,Chinese market only had re-usable glass syringe for humen injection.
1985
1995
Mr Zou Wenqing started the adhesive plaster and infusion set production.
Mr Zou Wenqing built a new workshop with another partner at Menghe Town,Changzhou city.
2004
2016
May.Under the leadership of our general manager Mr Zou Xiangjun,our brand new and mordern factory construction was launched at No.1 Dongtang Rd.,Zhenglu Town,Changzhou.After 2 years,we moved into the new workshop at Oct.,2018.
We passed TUV audit,and got the certificates of CE and ISO13485.
2018
2020
Oct.In order to protect the environment and reduce carbon emissions,we installed solar panels and the total installed capacity is 1.5 Megawatt.
March.Me started the FDA registration and will get 510K approval on the beginning of 2023.
2021
2022
April.We started the LDS(LDV) syringe production,this syringe can be used for vaccination injection.
Our founder Mr Zou Wenqing,started the disposable plastic syringe production,it's the earlist plastic syringe production line in China,but the workshop is not 100000 class medical grade.Before 1985,Chinese market only had re-usable glass syringe for humen injection.
1985
1995
Mr Zou Wenqing started the adhesive plaster and infusion set production.
Mr Zou Wenqing built a new workshop with another partner at Menghe Town,Changzhou city.
2004
2016
May.Under the leadership of our general manager Mr Zou Xiangjun,our brand new and mordern factory construction was launched at No.1 Dongtang Rd.,Zhenglu Town,Changzhou.After 2 years,we moved into the new workshop at Oct.,2018.
We passed TUV audit,and got the certificates of CE and ISO13485.
2018
2020
Oct.In order to protect the environment and reduce carbon emissions,we installed solar panels and the total installed capacity is 1.5 Megawatt.
March.Me started the FDA registration and will get 510K approval on the beginning of 2023.
2021
2022
April.We started the LDS(LDV) syringe production,this syringe can be used for vaccination injection.



 info@syringe.com
info@syringe.com